Neon has 8 valence electrons, which gives it a full shell so it cannot interact with outher elements. This is why it is called a noble gas. Pdf expert download for mac. A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons does an atom of Neon have? In the case of Neon the valence electrons is 0. Now let's check the facts about Neon. Neon Overview Neon Valence Electrons 0 Atomic Number 10 Learn more about the atomic number. The element that satisfies the given option should be chlorine with 7 valence electrons and 17 protons / 17 electrons (in total).Note: Oxygen has 6 valence electrons, neon has 8 valence electrons.
- The Number Of Valence Electrons In Neon Is
- How Many Valence Electrons In Neon
- Number Of Valence Electrons In Neon Family
- Valence Electrons Chart
Learning Objectives

- Define valence electron.
- Be able to indicate valence electrons when given the electron configuration for an atom.
What makes a particular element very reactive and another element non-reactive?
A chemical reaction involves either electron removal, electron addition, or electron sharing. The path a specific element will take depends on where the electrons are in the atom and how many there are.
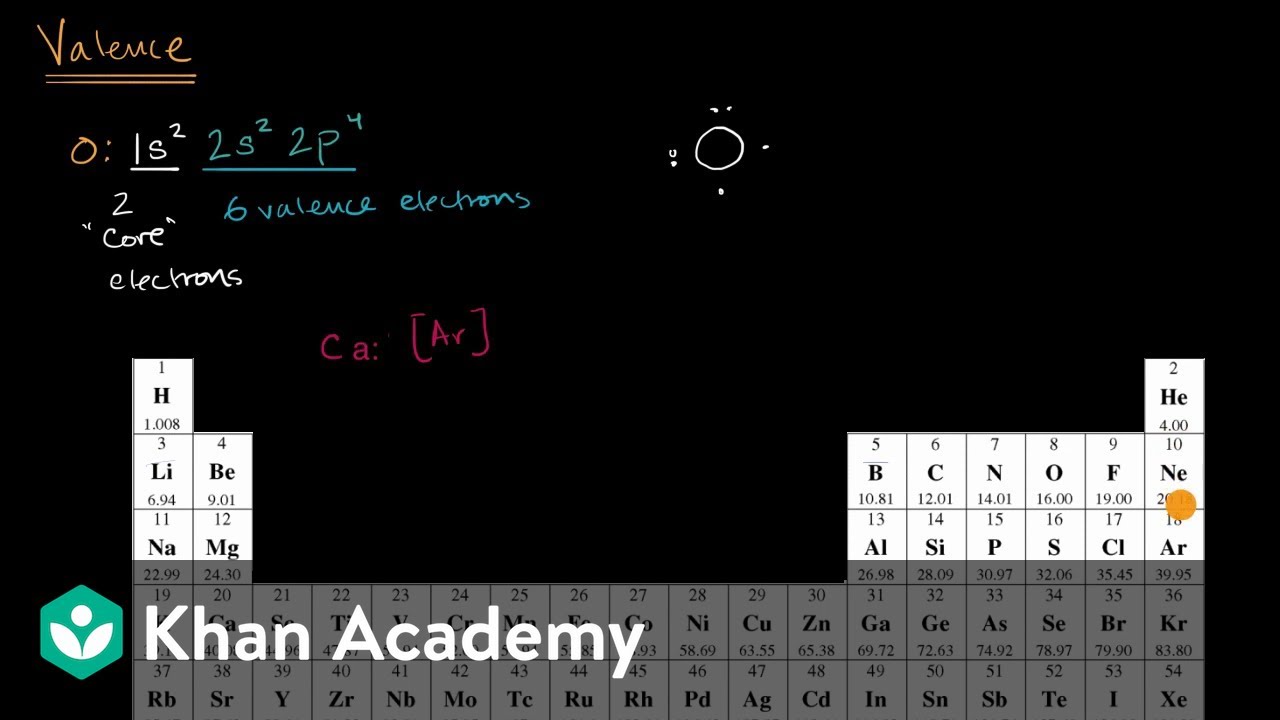
| Element Name | Symbol | Atomic Number | Electron Configuration |
| Lithium | Li | 3 | 1s22s1 |
| Beryllium | Be | 4 | 1s22s2 |
| Boron | B | 5 | 1s22s22p1 |
| Carbon | C | 6 | 1s22s22p2 |
| Nitrogen | N | 7 | 1s22s22p3 |
| Oxygen | O | 8 | 1s22s22p4 |
| Fluorine | F | 9 | 1s22s22p5 |
| Neon | Ne | 10 | 1s22s22p6 |
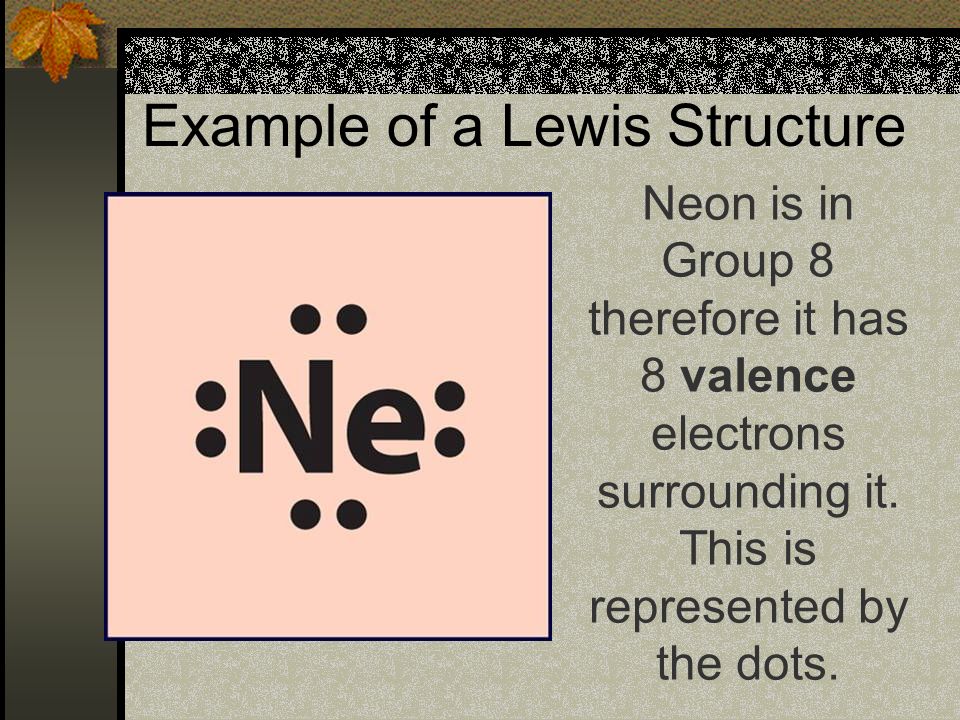
In the study of chemical reactivity, we will find that the electrons in the outermost principal energy level are very important and so they are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements listed above, the two electrons in the 1 s sublevel are called inner-shell electrons and are not involved directly in the element’s reactivity or in the formation of compounds. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the 2 s and the 2 p sublevels and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in s2p6, has eight valence electrons.
Summary
- Valence electrons are the outer-shell electrons of an atom.
- Valence electrons determine the reactivity of an atom.
The Number Of Valence Electrons In Neon Is
Practice
How Many Valence Electrons In Neon
Use the link below to answer questions about valence electrons:

Number Of Valence Electrons In Neon Family
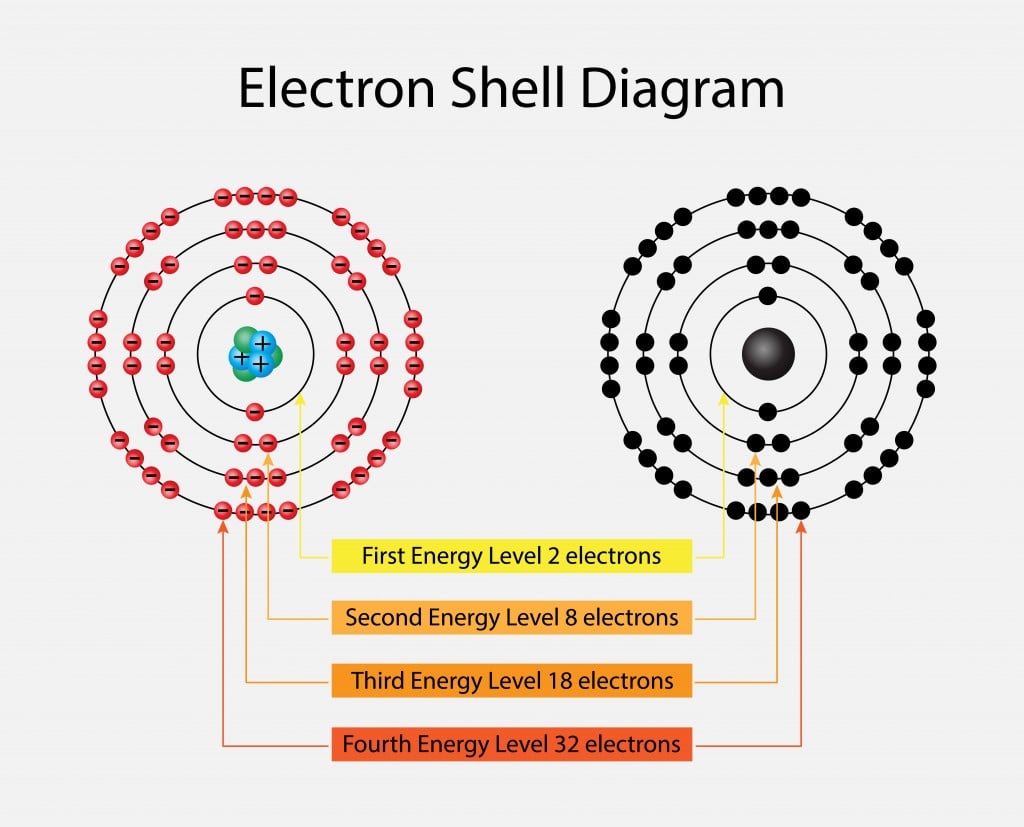
Review
- Define valence electron.
- Define inner shell electron.
- How many valence electrons are there in fluorine?
- What are the 2s electrons in nitrogen?
- How many inner shell electrons are there in beryllium?
Glossary
- inner-shell electrons: Those electrons that are not in the outer shell and are not involved in the reactivity of the element.
- valence electrons: The electrons in the highest occupied principal energy level of an atom.
Valence Electrons Chart
Show ReferencesReferences
- User:Chemicalinterest/Wikipedia. http://commons.wikimedia.org/wiki/File:Cobalt_carbonate.JPG.
